Our research group has developed synthetic methodologies allowing the C-H functionalization of various heteroaromatic compounds. We are particularly interested in the involvement of Pd nanoparticles as catalysts for these particular reactions. For example, we have demonstrated that piperidine lowers the reduction potential at Pd(II) leading to the more rapid generation of Pd nanoparticles in the C-H functionalization of nucelosides. We also have interests in studying the C-H activation reactions of 2-pyrones and related coumarins.
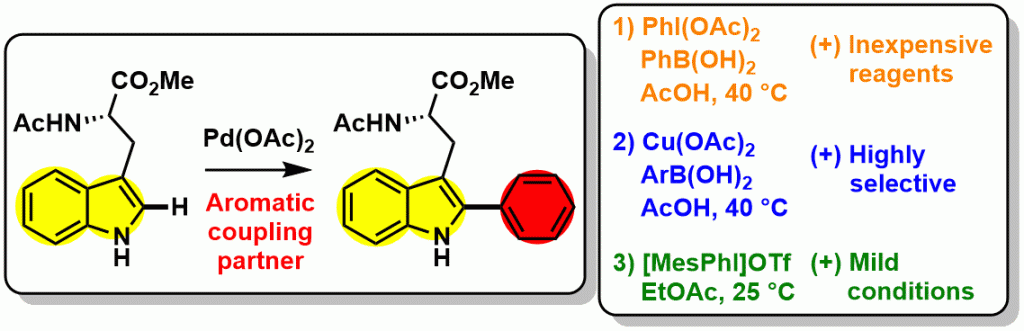
a) Arylation of amino acids, peptides and proteins
We are currently working on methodologies allowing the selective C-H bond functionalisation of amino acids such as tryptophan derivatives.
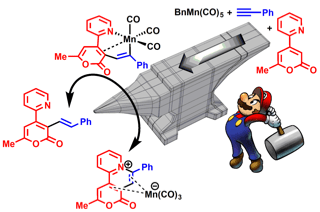
b) Mechanism of C-H bond activation involving manganese(I)
Over the last 5 years we have been examining the role of manganese(I) carbonyls in C-H bond activation reactions, using a combined experimental and computational approach. The project is in collaboration with Dr. Lynam and Simon Duckett.
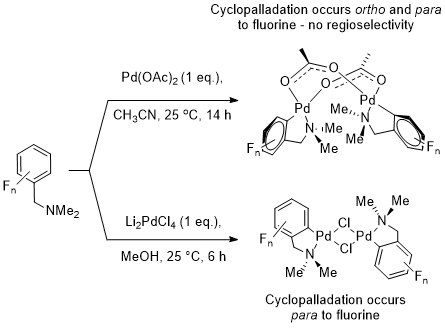
c) Mechanism of C-H bond activation at fluoroaromatics
We are currently working with Prof. Robin Perutz on mechanistic aspects of C-H bond activation involving fluorinated aromatic substrates. In our first joint paper together we revealed that there is no obvious ortho-fluorine effect in the cyclopalladation of suitable fluorinated benzyl amines. A difference is seen in products containing either bridging chloride or acetate ligands. For the former we see para-selective cyclopalladaton and for the latter we see no regiochemical preference.
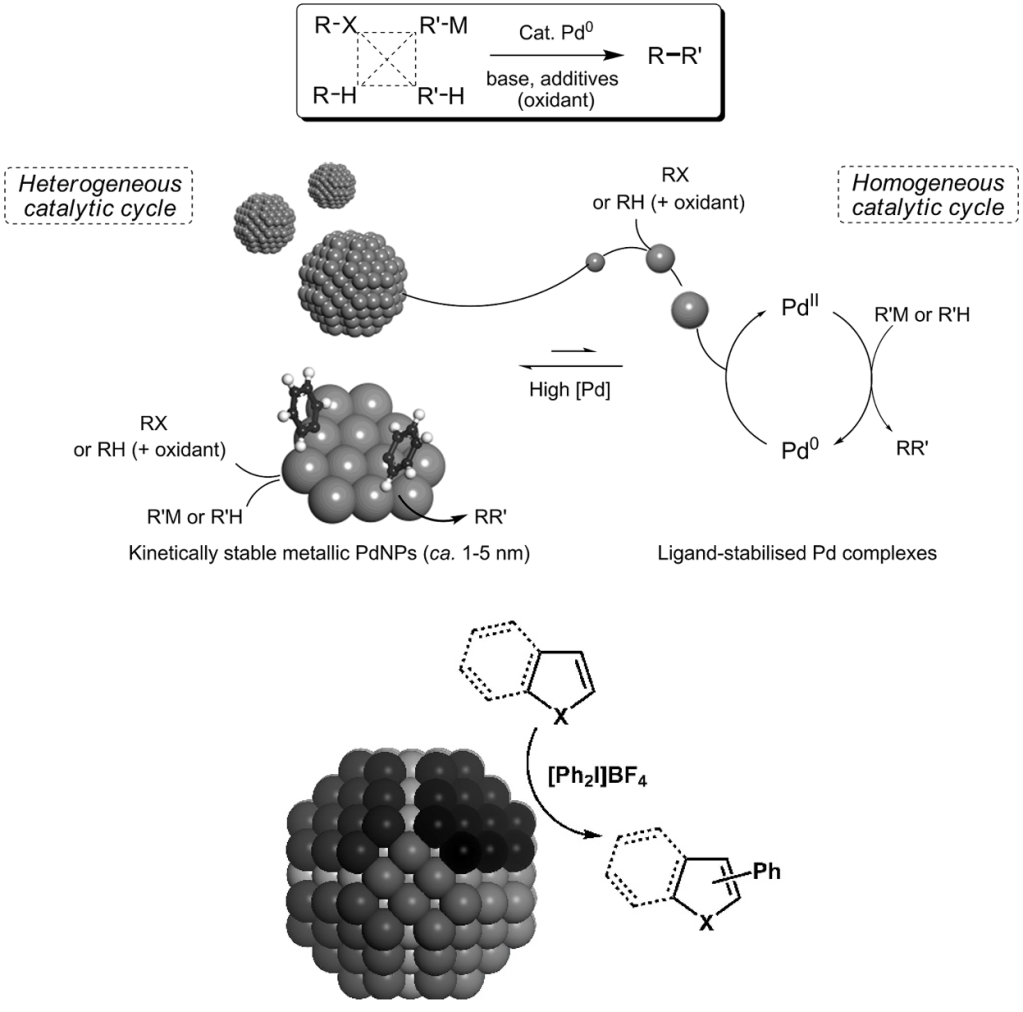
d) Involvement of Pd nanoparticles in C-H bond activation processes
We are currently exploring the role played by palladium nanoparticles in a variety of cross-coupling reactions. We are particularly interested in the formation of Pd clusters and the hybrid homogeneous-heterogeneous phase.
We have compared the catalyst efficacy of homogeneous and heterogeneous palladium catalysts in the direct arylation of common heterocycles. See, Synlett 2016, 27, 1211.
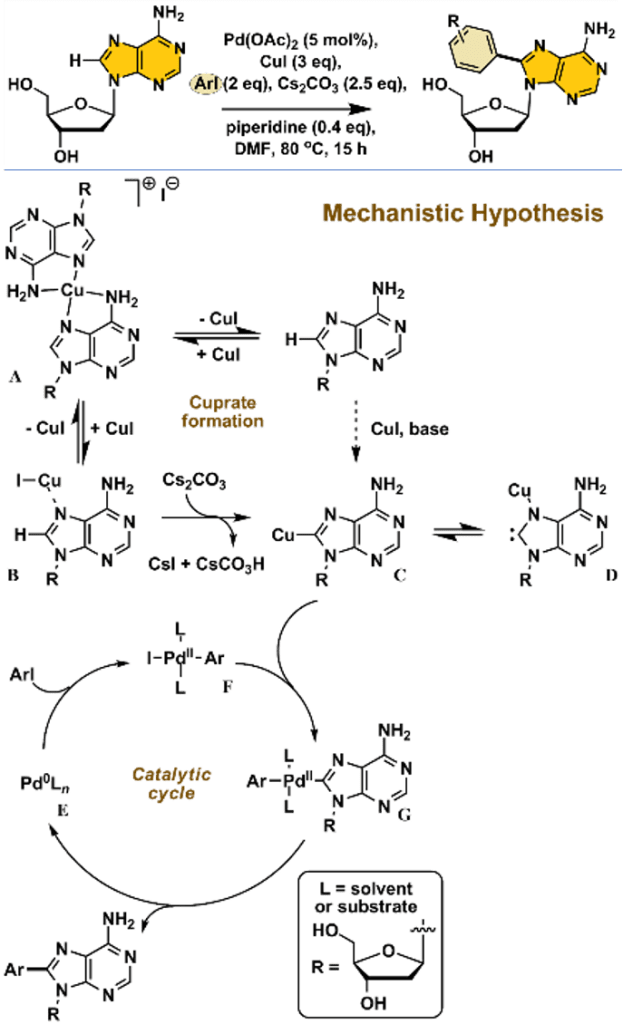
e) Catalytic C-H functionalisation in unprotected nucleosides:
C-modified non-natural nucleosides are used as fluorescent markers, light sensors, therapeutic agents, novel ligands for metals and supramolecular building blocks. These analogues allow biomolecular structure and biochemical mechanisms to be probed, which in turn provides the impetus to develop more efficient synthetic routes to structurally distinct purine nucleosides. In the reaction scheme opposite is shown an efficient approach which allows the arylation of 2′-deoxyadenosine selectively at the 8-position in the purine ring system. The process is mediated by a Pd(0) active catalyst species, most likely nanoparticulate, derived from the Pd(II) (pre)catalyst – “Pd(OAc)2“.
We are currently working on the mechanism of this transformation which is complex. Our working hypothesis is shown in the mechanistic scheme opposite. The requirement for stoichiometric Cu(I) is intriguing, as is the potential involvement of Cu-carbene intermediates C and D.
